
The Tysabri Patient Alert Card: available as an app for your smartphone
The Tysabri Patient Alert Card contains important safety information for you, your family & caregivers/healthcare professionals. You should always carry it with you, while you’re taking Tysabri and for 6 months after stopping Tysabri, and show it to any doctor involved in your treatment.
It’s available as an app for your smartphone, so you don’t have to carry paperwork with you.
Using your smartphone, scan the QR code or follow the link below to download the Tysabri Patient Alert Card app.

If you’re using an Android smartphone running Google Chrome:
Click the underlined link
If you’re using an Apple smartphone (iPhone) running Safari:
Long-press on the underlined link
Click ‘Open Link’
This app is produced by Biogen.
Installing the app onto your phone
If you’re using an Android smartphone running Google Chrome:
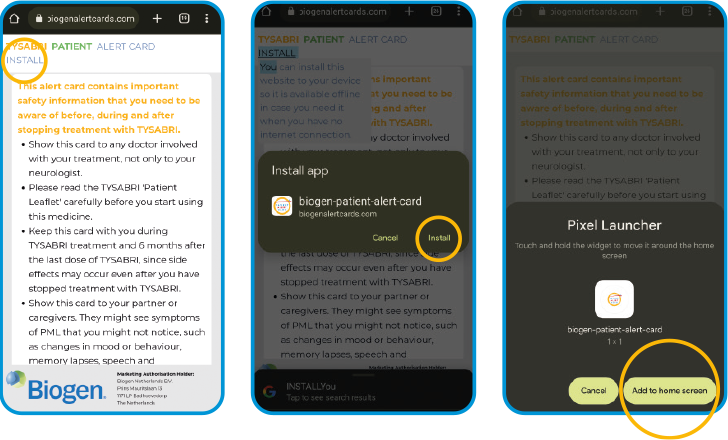
Click the ‘INSTALL’ link that appears at the top of your screen after scanning the QR code, then click ‘Install’ again
Then click ‘Add to home screen’, or follow the same steps you normally do to add the app to your home screen
If you’re using an Apple smartphone (iPhone) running Safari:
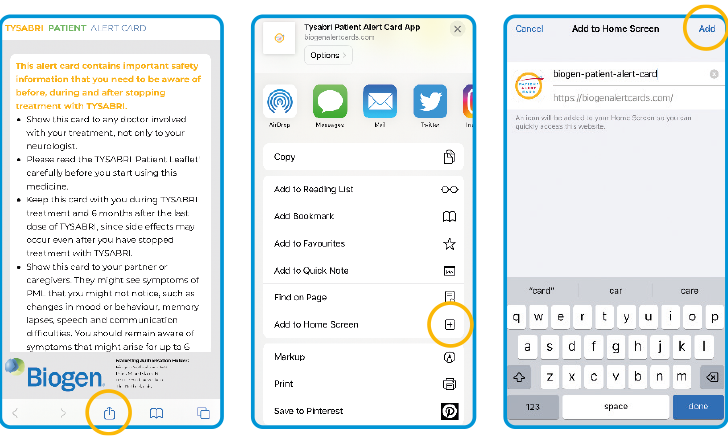
Click the download icon that can be dragged up from the bottom of your screen after scanning the QR code
Then scroll down, click ‘Add to Home Screen’ then ‘Add’
Once installed, the app will notify you when an update is available, so it can be shared to your smartphone.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any not listed in the package leaflet.
You can also report side effects directly via the national reporting system:
United Kingdom
Yellow Card Scheme
Website: https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store (UK).
Ireland
HPRA Pharmacovigilance, Earlsfort Terrace, Dublin, Ireland
Tel: +353 1 6764971
Fax: +353 1 6762517
Website: www.hpra.ie
Email: medsafety@hpra.ie
By reporting side effects you can help provide more information on the safety of this medicine.

